Background:
Multiple myeloma (MM) is a common global hematologic malignancy, contributing to substantial morbidity and mortality amongst diagnosed patients. Novel agents - bortezomib (Btz) and lenalidomide (Len) have changed dramatically over the past 20 years and have contributed to improvements in survival and long term outcomes for MM patients. Research has shown that there are differences in how MM is treated globally. We sought to evaluate the differences in outcomes and therapies received amongst patients treated in Alberta (AB), Canada, and in the United States (USA).
Methods:
Adult patients with multiple myeloma over the age of 65, diagnosed between 2007 and 2013, were identified. Data sources used for AB was the CA National Ambulatory Care Reporting System (NACRS), Discharge Abstracts Database (DAD), and CT records from AB Health Services. For the USA we used the SEER-Medicare database; MM diagnosis was extracted using a previously published algorithm. We extracted demographic data, novel agent utilization in the first year, comorbidities, and survival. Kaplan-Meier analysis was used to determine survival.
Results:
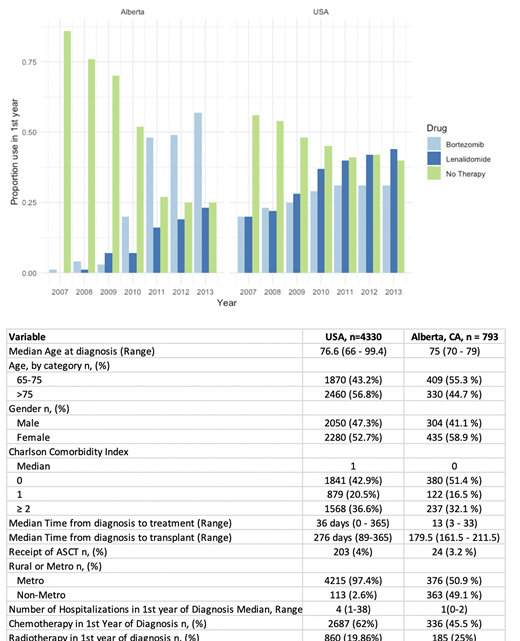
Patients meeting study criteria included 4,330 MM patients in the USA and 793 patients with MM in AB. The median age at diagnosis in the USA and AB was 76.6 and 75 years, respectively. Median Charlson Comorbidity index in the USA and AB was 1, and 0, respectively. With respect to frontline therapies, both AB and USA had increasing utilization of Btz and Len from 2007-2013 (Figure), with Btz use more common in AB, while Len use was more common in the US. The proportion of all patients who did not receive any therapy in the first year of diagnosis fell dramatically from 2007 to 2013 in both AB (from 86% to 25%) and the USA (from 56% to 40%). Receipt of autologous stem cell transplant was uncommon in this older cohort, in only 3.2% of MM patients in AB and 4% of patients in the US. Similar proportions of patients in the USA and AB received radiotherapy in the first year (19% US, 25% AB). Median overall survival was improved in AB at 2.8 years, (95% CI 2.6-3.2 yrs), compared with the USA, 2.2 yrs (95% CI 2.1 - 2.30, as was 5 year survival - 30% in the USA, compared with 34% in AB.
Conclusions:
In this cross-country comparison of MM patients in the USA and AB both treated in a single-payer health care system - AB Health Services and Medicare - novel agents are a common treatment in the first year of diagnosis. We demonstrate similar uptake of novel agents Btz and Len over 2007-2013, however, Btz utilization was higher in AB from 2011-2013, while Len was more often used in the USA from 2011-2013. Median overall survival was better in AB (2.8 years) compared with USA (2.3 years), as was long term survival at 5 years. The differences in utilization of therapy and survival are possibly related to variability in healthcare delivery systems, burden of comorbidities in these populations, and approval of anti-MM therapy over time, and require further study to better understand reasons for differential outcomes.
Cowan:Sanofi: Consultancy; Cellectar: Consultancy; Bristol Myers Squibb: Research Funding; Janssen: Consultancy, Research Funding; Abbvie: Research Funding. Ramsey:AstraZeneca: Other: Personal Fees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal